[ Prof. Gunsu Yun, Prof Dong Eon Kim] Quasi-equilibrium phase coexi…
- No attach File
관련링크
main text
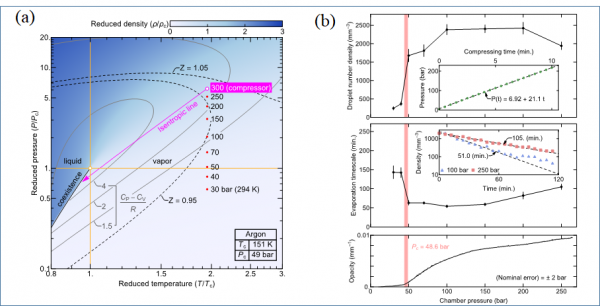
Caption: (a) Reduced phase diagram of argon. Large number of argon droplets are generated through compression-expansion cycles and survive over an hour in the supercritical background. (b) The number density, evaporation timescale and medium opacity by pressure. Micrometer-sized droplets and a few nanometer-sized clusters are generated.
Quasi-equilibrium phase coexistence in single component supercritical fluids
In their supercritical state simple fluids are generally thought to assume a homogeneous phase throughout all combinations of pressures and temperatures, although various response functions or transport properties may exhibit anomalous behavior, characterizing a state point as either more gas-like or liquid-like, respectively. While a large body of results has been compiled in the last two decades regarding the details of the supercritical phase in thermodynamic equilibrium, far less studies have been dedicated to out-of-equilibrium situations that nevertheless occur along with the handling of substances such as carbon dioxide or Argon. Here we consider successive compression-expansion cycles of equal amounts of Argon injected into a high-pressure chamber, traversing the critical pressure at two times the critical temperature. Due to expansion cooling, the fluid temporarily becomes sub-critical, and light scattering experiments show the formation of sub-micron-sized droplets and nanometer-scale clusters, both of which are distinct from spontaneous density fluctuations of the supercritical background and persist for a surprisingly long time. A kinetic rate model of the exchange of liquid droplets with the smaller clusters can explain this behavior. Our results indicate non-equilibrium aspects of supercritical fluids that may prove important for their processing in industrial applications.


-2.gif)







 Login
Login


